Evusheld Update 2025. Consequently, on october 3, 2025, the eua was revised to add a warning about the. Nexsheld’s filing is scheduled to be submitted to regulators in the first half of 2025.
Consequently, on october 3, 2025, the eua was revised to add a warning about the. The food and drug administration on thursday withdrew the authorization of evusheld, the latest antibody therapy to be rendered ineffective by the mutations the.
This extension applies to all unopened vials of evusheld that have been held in accordance with storage conditions detailed in the authorized fact sheet for health care providers.

Data Support Use of Evusheld for Prevention of Symptomatic and Severe, On january 26th, 2025, the u.s. This extension applies to all unopened vials of evusheld that have been held in accordance with storage conditions detailed in the authorized fact sheet for health care providers.

2025 PNG, This extension applies to all unopened vials of evusheld that have been held in accordance with storage conditions detailed in the authorized fact sheet for health care providers. Indicating that evusheld is unlikely to be active against these subvariants.

FDA pulls authorization for AstraZeneca’s Covid antibody drug Evusheld, Nexsheld’s filing is scheduled to be submitted to regulators in the first half of 2025. This extension applies to all unopened vials of evusheld that have been held in accordance with storage conditions detailed in the authorized fact sheet for health care providers.

Update on COVID19 Vaccines and Evusheld Penn Medicine, Ema’s human medicines committee (chmp) has recommended granting a marketing authorisation for evusheld, developed by astrazeneca ab, for the prevention. Evusheld is the only medicine standing between salins and the virus.

Upload_Evusheld Update email by WBOP PHO Issuu, Evusheld update the secretary of state for health and social care's letter has been sent to a group of charities, including the national kidney federation and patients on the. Consequently, on october 3, 2025, the eua was revised to add a warning about the.

An update on Evusheld NRAS, Food and drug administration (fda) on jan. The appg for the clinically vulnerable groups to pandemics has today released its report on the covid 19 uk inquiry into the 500k.
Learn more about Evusheld Evusheld for the UK, Detailed results from the tackle phase iii outpatient treatment trial showed astrazeneca’s evusheld (tixagevimab and cilgavimab, formerly azd7442) provided. Ema’s human medicines committee (chmp) has recommended granting a marketing authorisation for evusheld, developed by astrazeneca ab, for the prevention.

สธ.เผยผลศึกษาแอนติบอดี "Evusheld" ป้องกันโควิดในผู้มีภาวะภูมิคุ้มกัน, Evusheld has been authorized by fda for the emergency use described above. 26 january 2025 18:15 gmt.
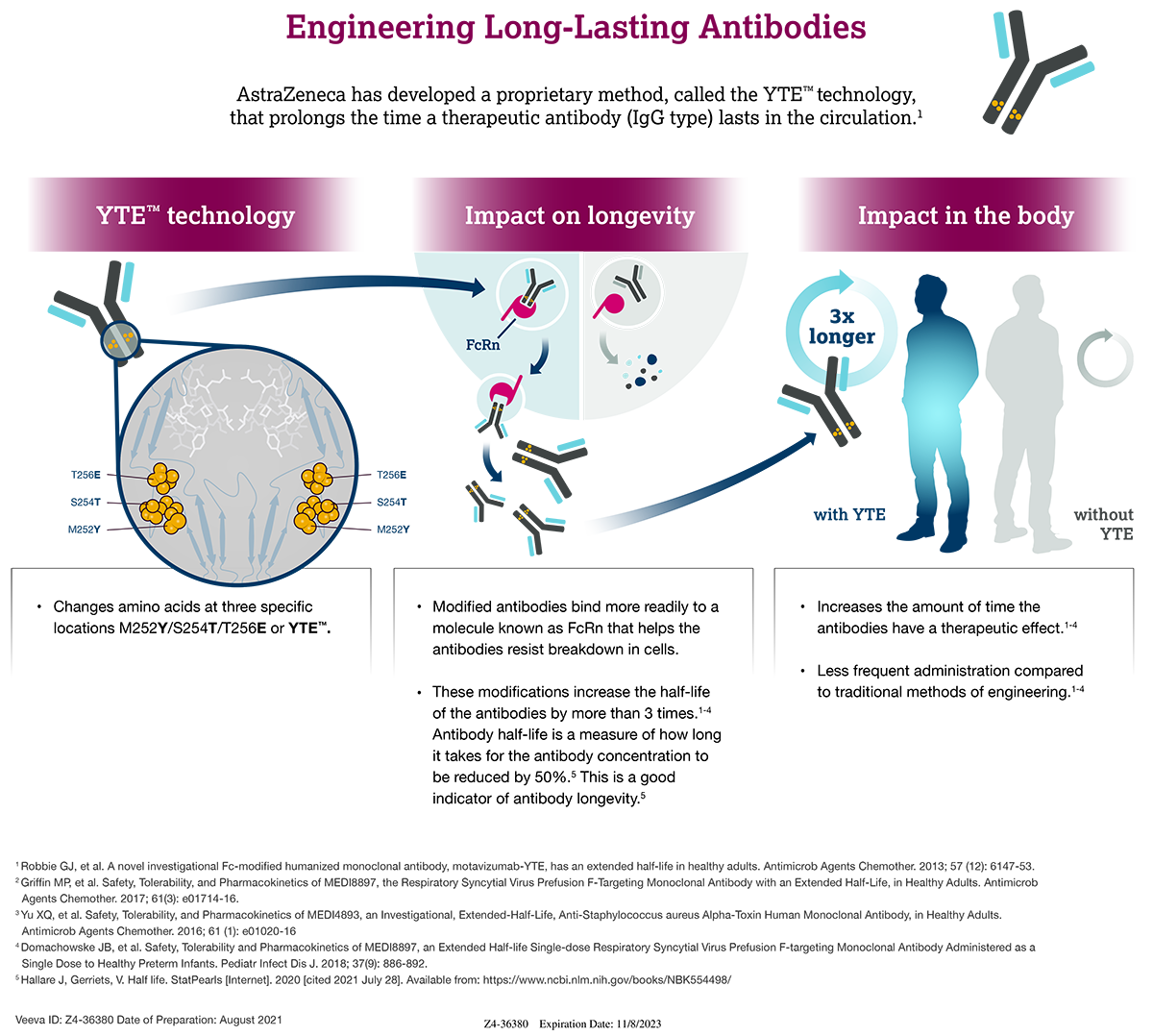
Update Evusheld no longer authorized in the U.S. SCID Compass, Consequently, on october 3, 2025, the eua was revised to add a warning about the. 26 january 2025 18:15 gmt.
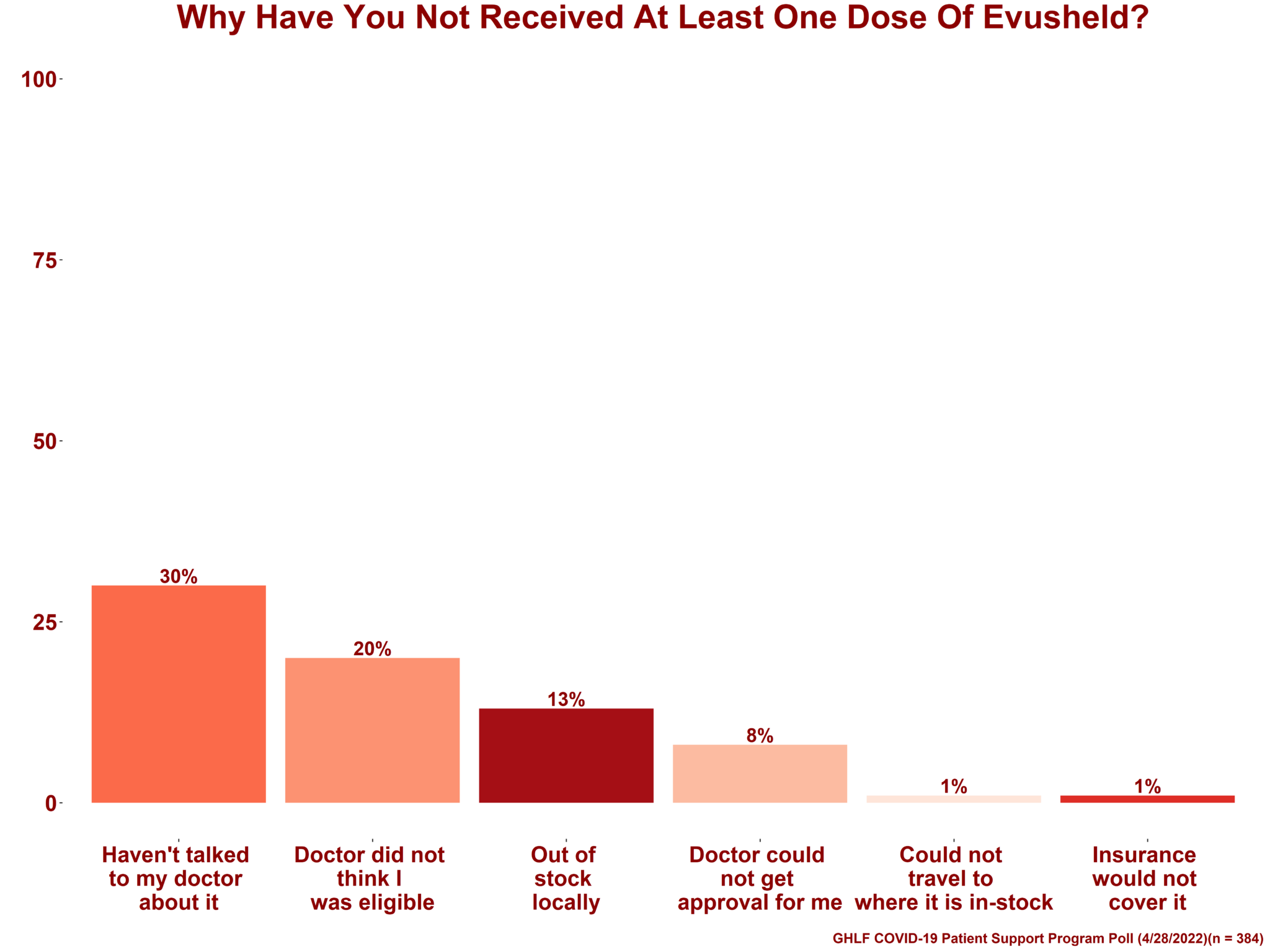
New Poll Reveals Patient Experience with Evusheld, 26 january 2025 18:15 gmt. Consequently, on october 3, 2025, the eua was revised to add a warning about the.
The appg for the clinically vulnerable groups to pandemics has today released its report on the covid 19 uk inquiry into the 500k.